Running Title: Cell Image Analysis for Esophageal
Dysplasia
Bin Zhou, Uta Jütting, Karsten Rodenacker,
Peter Gais, Pei-Zhong Lin
Bin Zhou is cytopathologist, guest scientist of Cancer Institute (Hospital), Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100021, China
Uta Jütting and Karsten Rodenacker are Diplom Mathematicians,
staff members and senior scientists, GSF-National Research Center
for Environment and Health, Institute for Biomathematics and Biometry,
D-85764 Oberschleißheim, Germany
Peter Gais is Diplom Engineer, staff member and senior scientist,
GSF-National Research Center for Environment and Health, Institute
of Pathology, D-85764 Oberschleißheim, Germany
Pei-Zhong Lin is Prof. of Cytopathology, Cancer Institute (Hospital),
Chinese Academy of Medical Sciences and Peking Union Medical College,
Beijing 100021, China
Address of correspondence: Uta Jütting, GSF-Institute
for Biomathematics and Biometry, Ingolstädter Landstr. 1,
D-85764 Oberschleißheim, Germany. Tel.: +49 89 3187 2555;
Fax: +49 89 3187 3127, e-mail: uta.juetting@gsf.de.
ABSTRACT
Objective: The principal
reason for the high resolution image analysis of esophageal dysplasia
is that of biomarkers of precancerous lesions which are urgently
needed as endpoints in the field of chemoprevention. Previous
studies have shown that there are around 25% esophageal epithelial
dysplasia in mass screening from 45-69 year old persons. But there
are about one third esophageal dysplasia patients develop to carcinoma
within several years. Some cases with dysplasia are static and
some regressing to normal. It is unable to discriminate one from
the other by cytopathologist under the microscope particularly
by discrimination of the nonspecific cell alternations and precancerous
lesions from the invasive. This paper tries to evaluate the way
of surrogate endpoints biomarkers for cancer incidence in chemoprevention
trials.
Study Design: Asymptomatic
adults were examined with balloon sampler in 1983 from Heshun
Commune of Linxian County. 50 cases of esophageal moderate dysplasia
and 68 cases of esophageal mild dysplasia were selected for this
study. By means of an Axiomat-microscope equipped with a TV-camera
100 visually normal intermediate squamous cell nuclei per specimen
were randomly measured from routinely Papanicoalou stained slides.
Results: Of 50 esophageal
moderate dysplasia cases 24 and 7 progressed to carcinoma within
3 and 9 years resp.. The other 19 cases remained stable or regressed
to nomal and were used as control group. By means of chromatin
features correct specimen classification rates of 79.2% (19/24)
and 73.7% (14/19) and 85.5% (6/7) and 84.2% (16/19) were achieved,
respectively (p<0.001).
Of 68 cases classified as mild dysplasia 16, 13 and
12 cases progressed to carcinoma within three, five and nine years,
resp.. The other 27 cases remained stable or regressed to normal
and were used as control group. The correct specimen classification
rates were 93.8% (15/16) and 88.9% (24/27), 69.2% (9/13) and 74.1%
(20/27), and 83.3% (10/12) and 77.8% (21/27) resp, using chromatin
features of the nuclei (p<0.001).
Conclusions: In this study
chromatin nuclear features measured by high resolution image analysis
can sufficiently good forecast the outcome of precancerous lesions
and discriminate precancerous lesions with progression and non-progression.
It also can be employed as surrogate endpoint biomarkers in clinical
chemoprevention trial. Stoechiometric staining and standard preparations
should increase the correct classification rates in our further
studies.
Keywords: high resolution image analysis, esophageal dysplasia,
surrogate endpoint biomarkers, chromatin features, malignancy
associated changes, MAC
INTRODUCTION
Esophageal cancer is a common disease with very poor
prognosis. Nearly 50 percent of the world's esophageal cancer
occurs in China (22), where it is the second leading cause of
cancer death after stomach carcinoma (16). The Taihang mountain
area of north-central China has recorded some of the highest esophageal
cancer mortality rates in the world. In Linxian, a high-risk county
in Henan province, the age-adjusted mortality rates for esophageal
cancer in 1973-1975 were 161 per 100,000 for men and 103 per 100,000
for women, and at age 75 there was a cumulative mortality from
esophageal cancer of over 20% for both sexes (15,16). Esophageal
cancer has been an important cause of death in this area for hundreds
of years.
Over the past 40 years, Chinese scientists have developed
esophageal balloon cytology (EBC) as an early detection technique
for identifying early cancerous lesions and for screening asymptomatic
precancerous lesions in high-risk populations (28, 29). Since
that time, systematic research work has been done on precancerous
lesions (also called intraepithelial neoplasia or dysplasia) of
the esophagus. Previous studies have reported that dysplasia of
esophageal epithelium is an essential step of esophageal carcinogenesis.
They are already validated as predictors of cancer incidence (17).
The conclusions suggest that, for the prevention of esophageal
carcinoma it is essential and possible to treat the precancerous
lesion by blocking its progression and promoting its regression
to normal (17). A large scale chemoprevention of esophageal carcinoma
study was launched in 1983. This trial enrolled 2531 men and women,
between 40 and 65 years old, who had no previous history of cancer
but cytologic evidence of esophageal dysplasia. The clinical chemoprevention
trial began in 1983 and ended in 1993. It was designed as a 2-arm
randomized placebo-controlled chemoprevention trial in which one
half of the participants received daily pills containing Anti
Tumour B (ATB, a pure Chinese traditional medicine) or Retinamide
and the other placebo pills. After 3 , 5 and 9 years, all living
participants were invited to have again a EBC procedure. More
than 90% accepted. Several months after these EBC exams, all individuals
with a EBC diagnosis of severe dysplasia were also invited to
undergo endoscope. The results indicated that ATB and Retinamide
have beneficial effects in reducing the progression of the esophageal
carcinognesis process and reduced the incidence rate of esophageal
carcinoma around 50% (p < 0.01), (18, 19).
As it is well known that esophageal epithelial dysplasia is an unsteady state, either developing to malignancy or regressing to normal. In fact, only a very small proportion of dysplasia will progress to cancer. Therefore, dysplasia is characterized by its capability to have two transformation pathways (17), however, even today, most of the morphological methods have a low degree of diagnostic specificity, somewhat subjective, and it is nearly impossible to get information on deeper layers of the cells. This is partially a result of difficulties in discriminating the cells of different lesions and of different biological behavior using morphological analogy, particularly of non-specific cell alterrations and precancerous lesions from invasive areas. It is a serious barrier to development of the field of chemoprevention as biomarkers. The biomarkers of precancerous lesions are urgently needed as endpoints in clinical trials of chemoprevention agents because they require less time, cost and effort compared to the conventional endpoint of cancer incidence reduction. It is highly desirable that early, precancerous lesions are identified which can serve as surrogate endpoints biomarkers (SEB) for cancer incidence in chemoprevention trials and, more importantly, as targets for chemoprevention (10). Obviously, the human cannot transform the visual impression of a nucleus into anything similar resembling a histogram, thus genuinely new information has to be gained for the diagnostic process. The high resolution image analysis may be able to appreciate very slight but consistent differences in nuclear appearance changes that are too subtle for a cytologist to observe.
MATERIALS AND METHODS
Subjects and Specimens:
Participants were recruited in the spring of 1983
from Heshun Commune of Linxian County, which is well-known for
its high rate of esophageal cancer. All of the 40-65 year olds
were asked to participate, unless they had a history of cirrhosis,
esophageal varices, vomiting blood, or were considered too weak
to undergo the examinations. 50 cases of esophageal moderate dysplasia
(a Chinese cytologic diagnosis of dysplasia I and II) and 68 cases
of esophageal mild dysplasia were selected for high resolution
image analysis (Table 1). During the next three and nine years,
there were 24 and 7 cases of moderate dysplasia progressed to
esophageal carcinoma. The other 19 cases remained stable or regressed
to normal. There were 16, 13 and 12 cases of mild dysplasia progressed
to esophageal carcinoma the next three, five and nine years, resp.
The other 20 cases remained stable or regressed to normal. The
diagnoses, the given class names, the number of specimens and
cells measured of each group are also listed in Table 1. All individuals
were followed up prospectively, with monthly visits by village
doctors, cytologic and endoscopic examinations of symptomatic
subjects, and additional cytologic screening examinations of selected
subjects in 1987 and 1993. Case records and diagnostic materials
(cytologic slides, histologic slides and x-rays) were reviewed
and the cancer diagnoses were confirmed by cytopathologists, pathologists
and radiologists from Cancer Institute, Chinese Academy of Medical
Sciences of China.
The balloon sampler used in this study was a rubber
balloon covered with a cotton mesh net and attached to a double-lumen
rubber tube. This balloon sampler has been used in most of the
mass population screenings performed in China during the last
40 years. After balloon technique, the slides were immediately
fixed in 95% ethanol for fifteen minutes, and later stained with
standard Papanicolaou stain.
Data acquisition:
By means of an Axiomat-microscope (Zeiss, Oberkochen,
Germany), equipped with a TV-camera (Bosch, T1VK9B1, Stuttgart,
Germany, 128x128 pixel) 100 visually normal well preserved intermediate
squamous cell nuclei per specimen were randomly selected and digitized.
The nuclei were scanned in transmission with a 100x objective
(oil immersion, numerical aperture 1.3) using an optical narrow
band filter of 548 nm wavelength. The pixel distance was 0.25
µm, and the nominal grey value resolution was covered by
256 channels. Image processing was carried out using a VAX 4000-500
processor (Digital, Maynard, USA) with software written under
idl (Interactive Data Language, RSI, Boulder, Colorado, USA).
Each nucleus was automatically segmented, visually controlled
and interactively improved if necessary. More than 100 quantitative
features (morphological, densitometrical, textural) were extracted
using the extinction or optical density image, which was derived
from transmission image (24). A shading correction was applied.
Using linear and non-linear filtering like Roberts gradient, Laplace
transform, flat texture image, local fractal and multi-fractal
dimensions, topological gradient, the difference of upper and
lower skeleton, and statistical features derived from runlength
and co-occurrence matrix (23, 27) several chromatin distribution
features were calculated from the whole nucleus as well as from
dark and bright regions of the nucleus. The latter were automatically
discriminated using the upper and lower skeleton which is similar
to the watershed algorithm applied to the extinction image and
its inversion (upper and lower skeleton). Due to nonstoechiometric
staining and data acquisition over years only those features have
been offered for classification that were proved to be nearly
independent of staining intensity, in order to avoid variances
due to preparation and staining influences (25). The remaining
feature set contained about 60 variables. In the appendix the
finally selected features are listed and shortly described.
Statistics:
All statistical evaluations were done using SAS (SAS Institute, Inc., Cary, North Carolina, USA) and BMDP (Statistical Software Inc., Los Angeles, CA, USA) program packages. All cells from specimens belonging to the same clinical sample were pooled and two-class stepwise linear discriminate analyses on cell level were applied. From the whole feature set only those features were offered in the classification steps which are univariate significant and not highly dependent from staining and preparation changes. Up to 10 features were stepwise selected on the basis of F-statistics. The value for the first chosen feature is the univariate one whereas the following F-values are multivariate reflecting the impact of results after using this feature together with the already selected features. For each specimen, the mean of the a posteriori probability (APOP) distribution of the corresponding cells was calculated. The APOP value and the double standard error of this mean (S.E.M.) were used for specimen classification. A specimen was classified into that class with the highest APOP value only if the mean APOP±S.E.M. did not cut a threshold (THR) which was set as the border between the two classes. In all cases, the threshold was defined at APOP=0.5 that is the half distance between both group means. Cases with THR{APOP±2S.E.M.} were called unclear (5). The significance for the specimen classifications was tested using contingency tables without defining unclear cases. In these cases and in the three-class discrimination the specimens were classified into that class with the highest APOP value. All statistical evaluations were done at 95% level.
Results
All investigations in the following concentrate on
the discrimination of cell nuclei from different defined dysplasia
classes and their progression status.
- Discrimination of patients with moderate dysplasia
with non-progression within 9 years (MODN9) and progression within
3 years (MODPC3)
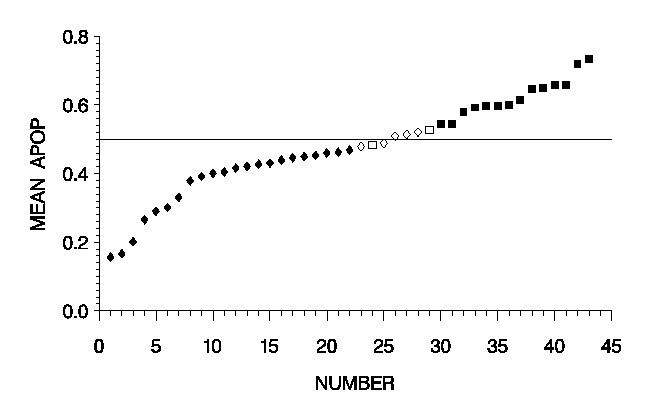
In this cell classification case the most important features were the number of dark particles (DNO), followed by DAA and HUM3. The cell classification rate was 58.5 % for the progression group and 61.9 % for the non-progression group. 15/24 specimens of the progression group and 10/19 of the non-progression group were correctly classified with 10 unclear (Table 1). This result is also shown graphically in Fig. 1. In case without unclear decisions 19 and 14 cases, resp. were correctly classified. This result is significant for p < 0.001. Fig. 2 demonstrates 6 nuclei with high values (30 pixels, upper row), representing cells from patients with progression and low values (10 pixels, lower row) of DNO representing cells from patients with non-progression whereas the nuclear size and the mean optical density are similar.
To test whether MODPC9 belongs better to MODPC3 or
to MODN9 all 7 specimens were classified as a testset according
to the evaluated discriminant function. One of the cases was classified
as MODPC3 the remaining as MODN9.
- Discrimination of patients with moderate dysplasia
with progression within 5-9 years (MODPC9) and non progression
within 9 years (MODN9)
By means of the subset of chromatin features, correct
cell classification rates of 63.2 % for MODPC9 and 64.6 % for
MODN9 were achieved. The best feature was NC13 which is the measure
of correlation 2 of co-occurrence of flat texture, followed by
RL2 and CO12. The subsequent specimen classification resulted
in 6/7 correct MODPC9 decisions and 12/19 correct MODN9 decisions,
4 cases were falsely classified (Table 3). In case of no unclear
decisions 4 cases remained falsly classified (p < 0.001).
- Three class discrimination of patients with
moderate dysplasia with progression within 3 years (MODPC3), within
5-9 years (MODPC9) and non progression within 9 years (MODN9)
The number of dark particles was the most important
feature to distinguish the three groups followed by DNOA and NC13.
14/24 MODPC3 specimens, all 7 of MODPC9 and 13 of MODN9 were correctly
classified. 3 of the first progression group was classified into
the non progression class MODN9 which is not favorable due to
non adequate treatment planning. The cell and specimen classification
results are listed in Table 4.
- Discrimination of patients with mild dysplasia
with non-progression within 9 years (MIDN9) and progression within
3 years (MIDPC3)
NR1, CO6 and HUSPAN were the most important chromatin
features to discriminate both classes. 71.3% of the nuclei of
MIDPC3 and 69.1 % of MIDN9 were correctly classified. The subsequent
specimen classification led to a classification rate of 87.5%
(14/16) and 81.5% (22/27) respectively with two and five unclear
decisions. Without the definition unclear cases 15/16 specimens
of MIDPC3 and 24/27 specimens of MIDN9 were correctly classified
(p<0.0001). The results are given in Table 5 and shown in Fig. 3.
- Discrimination of patients with mild dysplasia
with non-progression within 9 years (MIDN9) and progression within
3-5 years (MIDPC5)
A cell classification rate for MIDPC5 of 68.3% and
for MIDN9 of 60.4% were achieved using the chromatin features
NC3, NR1 and CO6. This led to a specimen classification of 8/13
(61.5%) and 18/27 (66.7%) respectively with 3 false decisions
and 11 unclear cases. The significance of this result without
unclear cases was p<0.01 (Table 6).
- Discrimination of patients with mild dysplasia
with non-progression within 9 years (MIDN9) and progression within
5-9 years (MIDPC9)
By means of the chromatin features MFRANG, MFM3 and
CO14 a cell classification rate of 60.8% for MIDPC9 and 69.0%
for MIDN9 could be achieved. The subsequent specimen classification
rate without definition of unclear cases was 83.3% (10/12) and
77.8% (21/27) respectively. This result is significant for p<0.001.
In Table 7 the specimen classifications are shown.
Summary of results:
These preliminary results suggest that using high resolution image analysis is a useful technique and highly sensitive for forecasting esophageal carcinoma in systematic patients. Our data shows that correct specimen classification rates of 72.5% to 90.7% could be achieved in case of mild and moderate dysplasia using chromatin features. These features can be applied to give hints for an individual treatment planning of each patient.
DISCUSSION
Esophageal cancer is a common malignancy with a very
poor prognosis. The main reason for that is that most cases are
asymptomatic until they are unresectable. Previous studies have
shown that cancer chemoprevention has beneficial effects in reducing
the progression of the esophageal carcinogenesis process and reduced
the incidence rate of esophageal carcinoma around 50%. At present
time, cancer chemoprevention is a rapidly expanding area of oncology.
But it will be emphasized that clinical trials of chemoprevention
require the unacceptable cost (millions of dollars), long duration
(5-10 years), and large scale of effort (thousands of subjects)
(7).
Even today, it is nearly impossible to get information
on the deeper layers of the cells using the morphological analogy.
This is partially a result of difficulties in discriminating the
cells of different lesions and biological behavior, particularly
in the discrimination of the nonspecific cell alternations and
precancerous lesions from the invasive. In this setting, there
is a clear need to develop practicable endpoint biomarkers in
clinical trials of chemoprevention agents.
In the recent years, many publications on the subject of cell image analysis have appeared in the literature. But using high resolution image analysis to discriminate dysplasia with progression and non-progression is not well documented.
In 1966 Mendelsohn suggested that the recording of
high resolution images and subsequent analysis by a digital computer
could lead to the automated recognition and classification of
five principal types of leukocytes (20). This classic paper applied
new concepts to microscopic image analysis and statistical classifications.
And then several authors initiated with the aim of objectivity
to clinical cytology (30). In 1980 and 1981 Wied and Burger (3,
31, 32) have noted such subvisual clue as marker features for
neoplastic events in the uterine cervix. Subtle changes in normal-appearing
epithelial cells adjacent to malignant tumors are called malignancy
associated changes (MAC) and can be detected by nuclear texture
measurements. And then others found that marker features were
of predictive value in assessing cases of moderate dysplasia in
routinely prepared cervical smears (26). Such marker features
have also been detected in other organ sites. Jahoda et al. (12,
13) correctly classified a very good result of normal and reactive
mesothelial cells, histiocytes, and cells of metastatic adenocarcinoma
of lung and breast origin in effusions and a high percentage of
mesothelial cells and ovarian cancer cells in peritoneal fluids.
Boon et al. (2) used image analysis to classify follicular adenoma
and carcinoma of the thyroid on aspiration smears. Hutchinson
et al. (11) achieved correct classification of prostate and breast
aspirates by image analysis. Many researchers have used objective
high resolution cytometry techniques to study precancerous lesions
and malignant diseases from a wide variety of body sites. i.e.
renal cell carcinoma (1), nevi and melanomas (8), lung (21) buccal
mucosa (14), nasal mucosa (22) and head and neck (4). It is clear
that image cytometry is capable of providing diagnostically and
prognostically relevant information regarding disease. Particularly
mentioned that F. Gao et al. (9) got surprisingly good results
which used high resolution image analysis to discriminate esophageal
severe dysplasia with progression and non-progression.
In recent years, some scientists emphasize and believe
that use high resolution cytometric biomarkers of precancerous
lesions which are urgently needed as endpoints in clinical trials
of chemopreventive agents because they require less time, money
and effort compared to the conventional endpoint of cancer incidence
reduction (6, 7, 21).
In this study of 50 esophageal smears representing
moderate dysplasia and 68 cases of mild dysplasia, it is shown
that the progression to carcinoma within several years and non-progression
can be discriminated using texture features (Table 2-7). Nearly
75% of the moderate dysplasia and about 80% mild dysplasia can
be correctly classified by means of benign looking intermediate
cells. We found that the behavior of both dysplasia types are
similar. That is to say that some dysplasia lesions are not real
precancerous lesions and are non-specific cell alternations. It
is why that only a small proportion of dysplasia will progress
to cancer, a majority of these lesions will spontaneously regress
or stable. Our results have also shown that there has been little
differences between three years and longer time of dysplasia with
progression. We noticed similar classification rates for mild
and moderate dysplasia discriminations. It is shown that even
cells with subtle changes can be detected by high resolution image
analysis.
To date, efforts at early detection of esophageal
cancer and precancerous lesions have concentrated on cytologic
or histologic categorization. During this period, nearly all dysplasia
patients made diagnosis by cytologic and histologic criteria in
clinical chemoprevention trial. But there was little possibility
to get information on the deeper layers of the precancerous lesion
cells by morphological methods. This has been proven to be difficult
when depending on morphological observation only, since many cases
have the same morphology but the biological behavior is quite
different. Our results show that the texture features can forecast
of precancerous lesions and can also be employed as surrogate
endpoint biomarkers.
In prospective studies the preparation technique and staining conditions have to be standardized to avoid unexpected results. We believe that high resolution image analysis, which is a powerful weapon of cytology diagnosis and research can provide us with important information which is impossible to be observed through routine methods. Cell measurement of the texture features improves cytological diagnosis and could be used to monitor progression of lesions as well as the treatment.
REFERENCES
(1) Bibbo, M., Galera-Davidson, H., Dytoh, H.E.,
Gonzalez de Chaves, J., Lopez-Garrido, J., Bartels, P.H., Wied,
G.L., Karyometry and histometry of renal-cell carcinoma, Anal.
Quant. Cytol. Histol., 9: 182-187, 1987
(2) Boon, M.E., Trott, P.A., Kaam, H.V., Kurver,
P.H.J., Baak, J.P.A., Computation of preoperative diagnosis probability
for follicular adenoma and carcinoma of the thyroid on aspiration
smears, Anal. Quant. Cytol. Histol., 4: 1-5, 1982
(3) Burger, G., Jütting, U., Rodenacker, K.,
Changes in benign cell populations in cases of cervical cancer
and its precursors, Anal. Quant. Cytol., 3: 261-271, 1981
(4) Burger G, Aubele M, Clasen B, Jütting U,
Gais P, Rodenacker K: Malignancy associated changes in squamous
epithelium of the head and neck region. Analyt Cell Pathol 7:181-193,
1994
(5) Burger G, Jütting U: Specimen classification
in cytometry: an intercomparison of various means of decision
making. In: Gelsema ES, Kanal LN, eds. Pattern recognition in
Practice II. Amsterdam: North-Holland Publ., 509-519, 1985
(6) Boone Ch, Kelloff G, Interaepithelial Neoplasia,
Surrogate endpoint biomarkers, and cancer chemoprevention, J.
Cellular Biochemistry, Supplement 17F: 37-48,1993
(7) Boone Ch, Kelloff G: Development of surrogate
endpoint biomarkers for clinical trials of cancer chemopreventive
agents: Relationships to fundamental properties of preinvasive
(Intraepithelial) neoplasia, Journal of Cellular Biochemistry,
Supplement 19: 10-22, 1994
(8) Fleming, M.G., Wied, G.L., Dytch, H.E., Image
analysis cytometry of dysplastic nevi, J. Invest. Dermatol.,
95: 287-291, 1990
(9) Gao F, Jütting U, Rodenacker K, Gais P,
Lin P-Z, Relevance of chromatin features in the progression of
esophageal epithelial severe dysplasia, Analyt. Cell. Pathol.
13: 17-28, 1997
(10) Kelloff G, Boone Ch, Steele V, Crowell JA, Lubet
R, Sigman C, Progress in cancer chemoprovention: Perspectives
on agent selection and short-term clinical intervention trials,
Cancer Research (Suppl) 54: 2015s-2024s, 1994
(11) Hutchinson, M.L., Schultz, D.S., Stephenson,
R.A., Wong, K.L., Harry, J., Zahnizer, D.J., Computerized microscopic
analysis of prostate fine needle aspirates: Comparison with breast
aspirates, Anal. Quant. Cytol. Histol., 11: 105-110, 1989
(12) Jahoda, E., Bartels, P.H., Bibbo, M., Bahr,
G.F., Holzner, J.H., Wied, G.L., Computer discrimination of cells
in serous effusions. I. Pleural Fluids, Acta Cytol., 17: 94-105,
1973
(13) Jahoda, E., Bartels, P.H., Bibbo, M., Bahr,
G.F., Holzner, J.H., Computer discriminations of cells in serous
effusions. II. Peritoneal Fluid, Acta Cytol., 17: 533-537,
1973
(14) Klawe, H., Rowinsk, J.: Malignancy Associated
Change (MAC) in cells of buccal smears detected by objective image
analysis, Acta Cytol. 18: 30-33, 1974
(15) Li, J.Y.: Epidemiology of esophageal cancer
in China, Natl. Cancer Inst. Monogr., 62: 113-120, 1982
(16) Li, M.X., Li, B., Li, B.R., Recent progress
in research on esophageal cancer in China, Adv. Cancer Res.,
33: 173-249, 1980
(17) P.Z. Lin, Introduction of research works in
esophageal precancerous lesions (dysplasia of esophageal epithelium),
Chinese Journal of Cancer 5: 391, 1983
(18) P.Z. Lin, J.S. Zhang, Z.P. Rong, R. Han, S.P.
Xu, R.Q. Gao, S.G. Cao, Second line prevention from esophageal
cancer inhibitory therapy to block precancerous lesions, Chinese
Journal of Cancer Research 1: 37-46, 1988
(19) P.Z. Lin, J.S. Zhang, Z.P. Rong, R. Han, S.P.
Xu, R.Q. Gao, Z.W. Ding, J.X. Wang, H.J. Feng, S.G. Cao, Studies
on medicamentous inhibitory therapy for esophageal precancerous
lesions 3- and 5- year inhibitory effects of Antitumor-B, Retinamide
and Riboflavin, Proc CAMS and PUMC, 5: 121-129, 1990
(20) Mendelsohn, M.L., Mayall, B.H., Prewitt, J.M.S.,
Bostrom R.S., Holcomb, W.G.: Digital transformation and computer
analysis of microscopic image. In: Barer, R., and Cosslett, V.E.
(eds.): Advances in Optical and Electron Microscopy. New York,
Academic Press, 1968
(21) Palcic B.: Nuclear texture: Can it be used
as a surrogate endpoint biomarker? J. Cellular Biochemistry,
Supplement 19: 40-46, 1994
(22) Parkin, D.M., Pisani, P., Ferlay, J.: Estimates
of the worldwide incidence of eighteen major cancer in 1985,
Int. J. Cancer 54: 594-606, 1993
(23) Reith A.K, Reichborn-Kjennerud S., Aubele M,
Jütting U, Gais P, Burger G, Biological monitoring of chemical
exposure in nickel workers by image cytometry (ICM) of nasal smears,
Analyt. Cell. Pathol. 6: 9-21, 1994
(24) Rodenacker K: Featuring of cellular objects.
In: Burger G, Ploem JS, Goerttler K, eds. Clinical Cytometry and
Histometry. Rep Part 3 4:73-78, 1973
(25) Rodenacker K: Invariance of textural features
in image cytometry under variantion of size and pixel magnitude.
Analyt Cell Pathol 8:117-133, 1995
(26) Rosenthal, D.L., Philippe, A., Hall, T.L.,
Harami, S., Missirlian, N., Suffin, S.C., Prognosis of moderate
dysplasia. Predictive value of selected markers in routinely prepared
cervical smears, Anal. Quant. Cytol. Histol., 9: 165-168, 1987
(27) Schmidt J, Aubele M, Jütting U, Rodenacker
K, Luz A, Erfle V, Burger G: Computer-assisted imaging cytometry
of nuclear chromatin reveals bone tumor virus infection and neoplastic
transformation of adherent osteobast-like cells. Biochem Biophys
Res Commun 164:728-735, 1989
(28) Shu, Y.J., The cytopathology of the esophagus;
an overview of esophageal cytopathology in China, Acta Cytol.,
27: 7-16, 1983
(29) Shu, Y.J., The Cytopathology of Esophageal Carcinoma,
Masson, New York 1985
(30) Wied, G.L., Bahr, G.F., Oldfield, D.G., Bartels,
P.H., Computer-assisted identification of cells from uterine adenocarcinoma.
A clinical feasibility study with TICAS, Acta Cytol. 12: 357-370,
1968
(31) Wied, G.L., Bartels, P.H., Bibbo, M., Sychra.,
J., Cytomorphometric markers for uterine cancer in intermediate
cells. Anal. Quant. Cytol., 2: 257-263, 1980
(32) Wied, G.L., Bartels, P.H., Dytch, H.E., Pishotta,
F.T., Yamauchi, K., Bibbo, M., Diagnostic marker features in dysplastic
cells from the uterine cervix, Acta Cytol., 26: 475-483, 1982
Table 1: Cytology results from the 1983 esophageal
balloon cytology surveys in Linxian, China, the follow up results,
and number of specimens and measured cell nuclei
|
Number of cases/ (cells) - Progression to cancer within 3 years Progression to cancer within 3-5 years Progression to cancer within 5-9 years Non-progression to cancer within 9 years |
- - PC3 - - - PC9 - N9 - |
MOD 50 (5474) 24 (2641) - NA - 7 (755) - 19 (2078) - |
MID 68 (7603) 16 (1798) - 13 (1449) - 12 (1337) - 27 (3019) - |
- - PC3 - PC5 - PC9 - N9 - |
NA: specimens not available
Table 2: Specimen classification results from moderate
dysplasia with progression within 3 years (MODPC3) and non progression
within 9 years (MODN9)
Specimen classification:
|
- MODPC3 MODN9 |
MODPC3 15 4 |
MODN9 4 10 |
unclear 5 5 |
total 24 19 |
% correct 62.5 52.6 |
Specimen classification:
|
- MODPC3 MODN9 - |
MODPC3 19 5 - |
MODN9 5 14 - |
total 24 19 - |
% correct 79.2 73.7 76.7 |
p < 0.001
Selected features with F-values:
DNO (83.5), DAA (83.5), HUM3 (27.7), HUCV (22.8),
GCV ( 15.6)
Table 3: Specimen classification results from moderate
dysplasia with progression within 7 to 9 years (MODPC9) and non
progression within 9 years (MODN9)
Specimen classification:
|
- MODPC9 MODN9 |
MODPC9 6 - |
MODN9 - 12 |
unclear 1 7 |
total 7 19 |
% correct 85.7 63.2 |
Specimen classification:
|
- MODPC9 MODN9 - |
MODPC9 6 3 - |
MODN9 1 16 - |
total 7 19 - |
% correct 85.5 84.2 84.6 |
p < 0.001
Selected features with F-values:
NC13 (78.4), RL2 (25.4), CO12 (31.9), HUCV (37.6),
HLM3 ( 35.1)
Table 4: Cell and specimen classification results
from moderate dysplasia in the 3 class case
Cell classification:
|
- MODPC3 MODC9 MODN9 |
MODPC3 1123 115 567 |
MODC9 753 429 597 |
MODN9 765 211 914 |
total 2641 755 2078 |
% correct 42.5 56.8 44.0 |
Specimen classification:
|
- MODPC3 MODC9 MODN9 - |
MODPC3 14 - 5 - |
MODC9 7 7 1 - |
MODN9 3 - 13 - |
total 24 7 19 - |
% correct 58.3 100.0 68.4 68.0 |
p < 0.001
Selected features with F-values:
DNO (67.1), DNOA ( 91.1), NC13 (26.8), GCV, (21.1),
HAA (20.3)
Table 5: Specimen classification results from mild
dysplasia cases with progression within 3 years (MIDPC3) and non
progression within 9 years (MIDN9)
Specimen classification:
|
- MIDPC3 MIDN9 |
MIDPC3 14 - |
MIDN9 - 22 |
unclear 2 5 |
total 16 27 |
% correct 87.5 81.5 |
Specimen classification:
|
- MIDPC3 MIDN9 - |
MIDPC3 15 3 - |
MIDN9 1 24 - |
total 16 27 - |
% correct 93.8 88.9 90.7 |
p < 0.0001
Selected features with F-values:
NR1, (492.3), CO6 (165.7), HUSPAN, ( 69.6), NC8 (
80.9), FRCV (83.7)
Table 6: Specimen classification results from mild
dysplasia cases with progression within 3-5 years (MIDPC5) and
without progression within 9 years (MIDN9)
Specimen classification:
|
- MIDPC5 MIDN9 |
MIDPC5 8 2 |
MIDN9 1 18 |
unclear 4 7 |
total 13 27 |
% correct 61.5 66.7 |
Specimen classification:
|
- MIDPC5 MIDN9 - |
MIDPC5 9 7 - |
MIDN9 4 20 - |
total 13 27 - |
% correct 69.2 74.1 72.5 |
p < 0.01
Selected features with F-values:
NC3 (272.5), NR1 (63.2), CO6 (79.3), HCV (33.2),
MM1 (20.1)
Table 7: Specimen classification results from mild
dysplasia cases with progression within 5-9 years (MIDPC9) and
non-progression within 9 years (MIDN9)
Specimen classification:
|
- MIDPC9 MIDN9 |
MIDPC9 7 4 |
MIDN9 2 18 |
unclear 3 5 |
total 12 27 |
% correct 58.3 66.7 |
Specimen classification:
|
- MIDPC9 MIDN9 - |
MIDPC9 10 6 - |
MIDN9 2 21 - |
total 12 27 - |
% correct 83.3 77.8 79.5 |
p < 0.001
Selected features with F-values:
MFRANG (259.6), MFM3 ( 48.2), CO14 ( 39.7), HUNO
(63.7), DNOA (28.3)
Appendix:
List of features and their description:
CO6 Sum average of co-occurrence of extinction
CO12 Measure of correlation 1 of co-occurrence of extinction
CO14 Local mean of co-occurrence of extinction
DAA Relative area of dark particles
DNO Number of dark particles
DNOA Relative number of dark particles
FRCV CV of local factal dimension
GCV CV of gradient filtered image
HAA Relative area of bright particles
HCV CV of extinction of bright particles
HLM3 Skewness of lower skeleton
HUCV CV of upper skeleton
HUM3 Skewness of upper skeleton
HUNO Number of bright regions (particles)
HUSPAN Range of upper skeleton
MFRANG Range of local multifractal dimension
MFM3 Skewness of local multifractal dimension
MM1 Morphological parameter (first invariant moment)
NC3 Correlation of co-occurrence of flat texture
NC8 Sum entropy of co-occurrence of flat texture
NC13 Measure of correlation 2 of co-occurrence of flat texture
NR1 Short runs emphasis of runlength distribution of flat texture
RL2 Long runs emphasis of runlength distribution
of flat texture
Figures